
“Curiouser and Curiouser!”
- Lewis Carroll -

RESEARCH
Molecular Imaging
The research performed in my lab focuses on developing novel diagnostic molecular imaging strategies that have great potential for clinical translation. These approaches are intended to improve cancer detection during routine screening and tumor resection with the hope of impacting the survival rate of cancer patients.

Molecular imaging provides the ability to study cellular and molecular processes. Molecular imaging differs from traditional imaging practices in that it utilizes contrast agents or imaging probes that interact with their surroundings either chemically or physically to produce a "functional" image of molecular processes. Several molecular imaging modalities that visualize these cellular and molecular processes central to cancer have already transformed cancer care. Some of these imaging modalities include positron emission tomography (PET), single photon emission computed tomography (SPECT), computed tomography (CT) and magnetic resonance imaging (MRI) and ultrasound.
More recently, several optical-based molecular imaging techniques have also begun to translate into the clinical setting for screening and surgical navigation including fluorescence, Raman and photoacoustic imaging. Each of these optical imaging techniques has its own unique advantages.
Fluorescence imaging is a well-established technique that offers real-time ultrasensitive wide field detection of fluorescent imaging probes with high quantum efficiency. In fact, there are several commercially available fluorescence image guidance tools that already exist in the clinic.
Raman imaging is a new molecular imaging technique that I have spent a considerable amount of time developing during my postdoctoral studies. It measures the inelastic scattering of light and offers unsurpassed multiplexing capabilities with resistance to both autofluorescence and photobleaching.
Photoacoustic imaging is a new technology that largely overcomes the depth and resolution limits of optical imaging. In photoacoustic imaging, light pulses excite target molecular imaging agents, causing very slight heat production. This produces ultrasound waves that are recorded by an ultrasound transducer that produces a three-dimensional image of the probe's distribution in living subjects.
Unlike the other molecular imaging modalities mentioned above, these optical imaging strategies tend to be more cost effective, utilize safer non-ionizing radiation, require less dedicated space and offer high resolution real time imaging with little disruption to the clinical setting/surgical suite. However, no single modality currently meets the clinical needs of high sensitivity, high spatial and temporal resolution, high multiplexing capacity, high depth of penetration, low cost, and high-throughput. Therefore, my lab is interested in developing a multimodal based imaging approach that combines the ultrasensitive wide field detection of fluorescence with the multiplexing capabilities of Raman and perhaps even adding the increased resolution and depth of penetration you get from photoacoustic imaging.
Fabrication of New Nano-based Contrast Agents
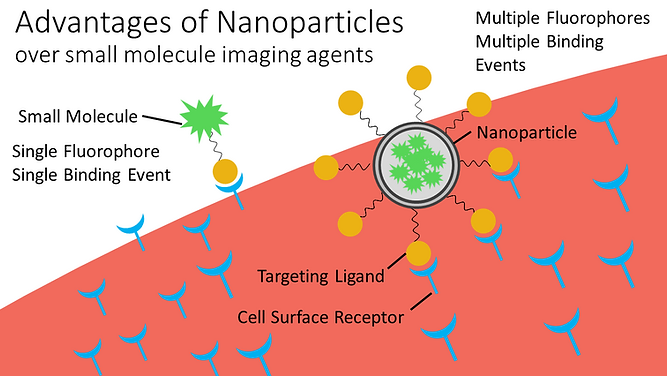
Nanoparticles are the perfect vehicle for molecular imaging contrast agents. Compared to their small molecule counterparts often used in clinics, nanoparticles can avoid renal filtration and improve tumor retention due to their larger diameter and mass.

Nanoparticles can exploit the leaky vasculature of solid tumors and passively accumulate in the tumor space due to the enhanced permeability and retention (EPR) effect. Unlike small molecule tumor targeting agents that display a single ligand, the large surface area of the nanoparticle allows for multiple tumor targeting ligands on their surface which increases the binding avidity and thus binding affinity of the imaging nano-probe to the tumor site. Therefore, several low binding ligands can work in tandem to create an accumulated strength that results in a high affinity nanoparticle construct for targeting multiple receptors on tumor cells.
Ongoing attempts toward developing new clinial nanoparticle-based contrast agents have faced major problems in gaining regulatory approval primarily due to their systemic toxicity and prolonged retention in the body. Our lab is developing novel approaches to overcome these regulatory barriers. We are investigating both biodegradable nanoparticles and alternative administration routes to deliver our nano-based imaging contrast agents.
Understanding Nanoparticle Physiology on a Microscopic Level in Real Time
Every nanoparticle construct is unique and their shape, size, and surface properties will often dictate their biodistribution patterns in the body. Before proceeding with tumor targeting studies and the clinical translation of these nano-based contrast agents, we must first fully characterize these newly developed nanoparticles in vivo. Therefore, my lab will utilize multiple non-invasive small animal molecular imaging modalities such as microPET and intravital microscopy (IVM) to evaluate both the macroscopic and microscopic biodistribution of each type of our newly developed nanoparticle constructs.
Our lab also intends to collaborate with Scott Fraser's group to utilize some of the multiphoton microscopes available within the Translation Imaging Center at USC. Compared to conventional single photon confocal microscopy, this multiphoton fluorescence microscopy imaging technique is superior offering low background signal, superior sensitivity, deeper tissue penetration and reduced phototoxicity. This powerful tool will enable real time microscopic visualization of our nanoparticles within the vasculature of a living mouse allowing us to evaluate the blood clearance or our nanoparticles and characterize important interactions of our nanoparticles with tumor vessel walls and their ability to extravasate into the tumor space, all in real time.
Several groups have reported that the size, shape and surface properties of a nanoparticle all influence their clearance rate, targeting efficiency and extravasation potential into tumors. The ability to monitor these parameters in real time could greatly impact the nano-imaging community and influence which nanoparticle constructs we take forward for clinical translation.
The Development of New Imaging Tools to Help Surgeons Identify Tumor Margins
Identifying tumor margins during surgical resection is a critical determinant of patient outcome. One of the biggest challenges faced by oncologic surgeons in the operating room (OR) is determining where the tumor they are resecting begins and ends. Obtaining negative tumor margins can be essential to the patient's survival. Current intraoperative surgical guidance depends largely on visual cues and tactile feedbacks, which may be highly subjective and distorted in diseased tissues. In addition to the negative oncologic consequences, positive tumor margins may necessitate reoperation, thereby increasing associated patient morbidity and healthcare expenditure (e.g. imaging, anesthesia, pathology)
Our lab is involved in the design, fabrication and characterization of new optical imaging tools for sensitively detecting tumor targeted nanoparticles in various solid tumors that require surgical excision.
Our team works closely with surgical oncologists to determine the optimal design for clinical use.
Live surgical resection of fluorescent LS174T tumor from adjacent normal tissue (dark background). Mouse had been IV injected with 200 nm G8 liposomes and euthanized 4 h post injection. Notice how the adjacent tissue does not show significant autofluorescence as compared to the neighboring tumor tissue, and the tumor is easily visualized with fluorescence image guidance.
Raman Topography Imaging

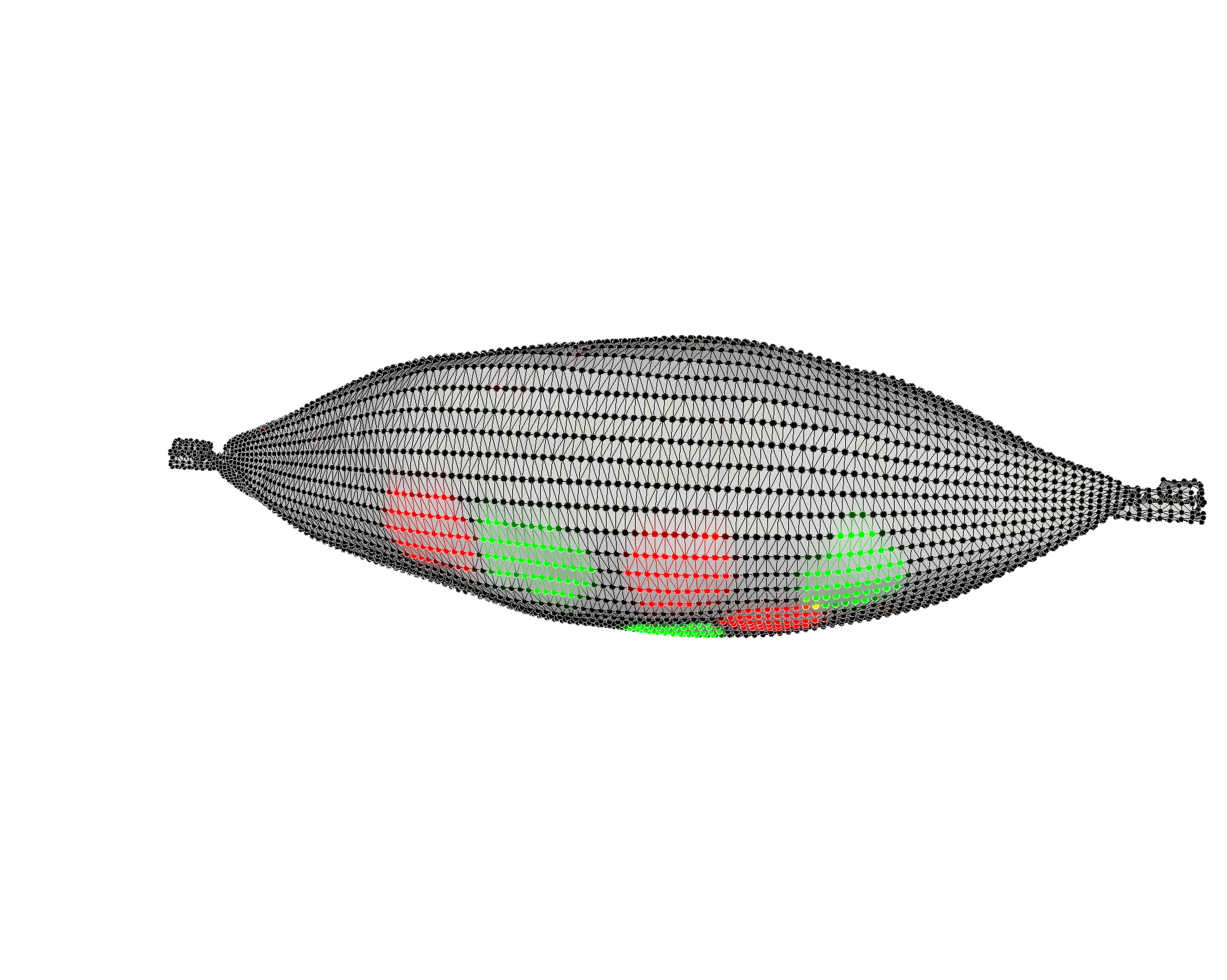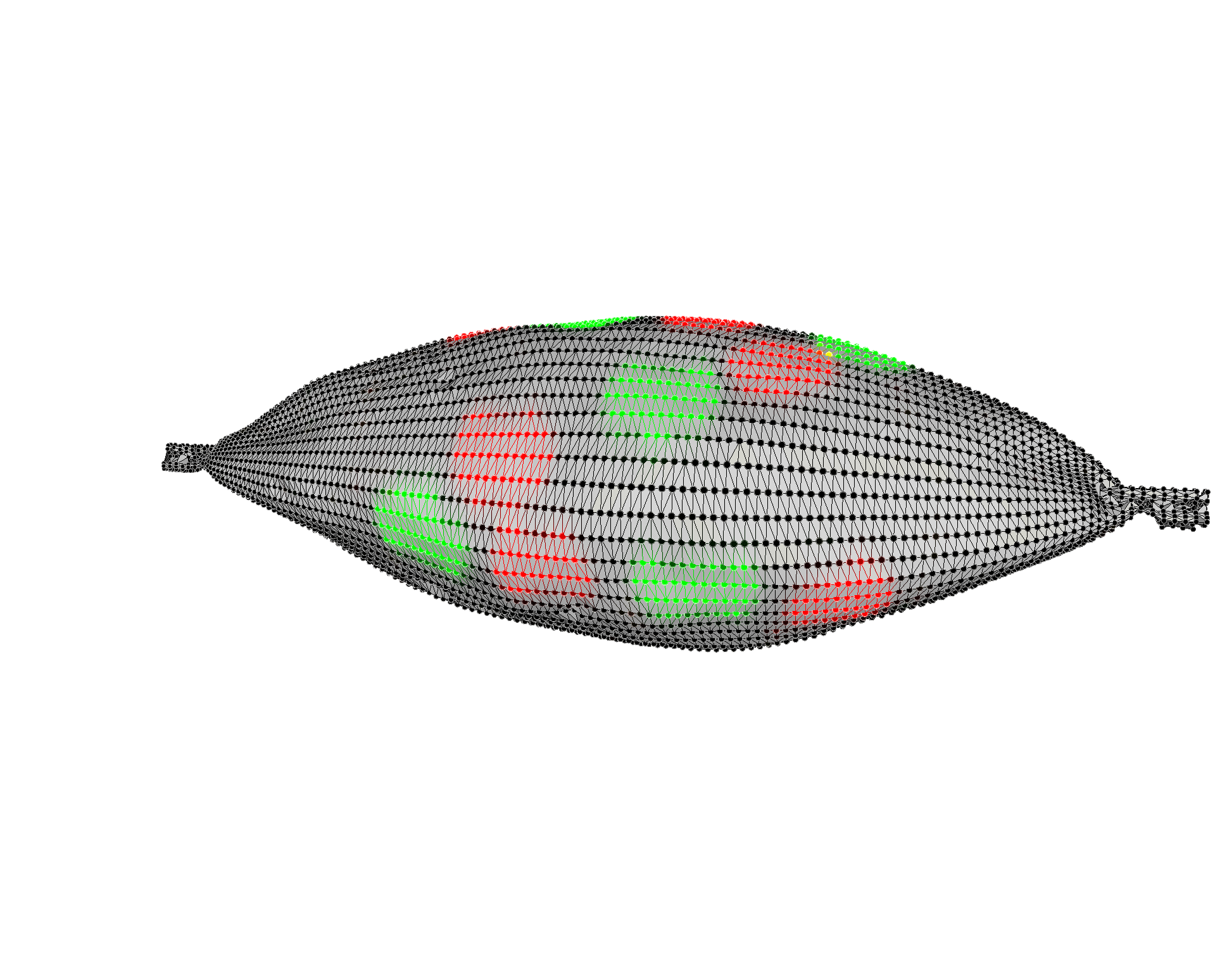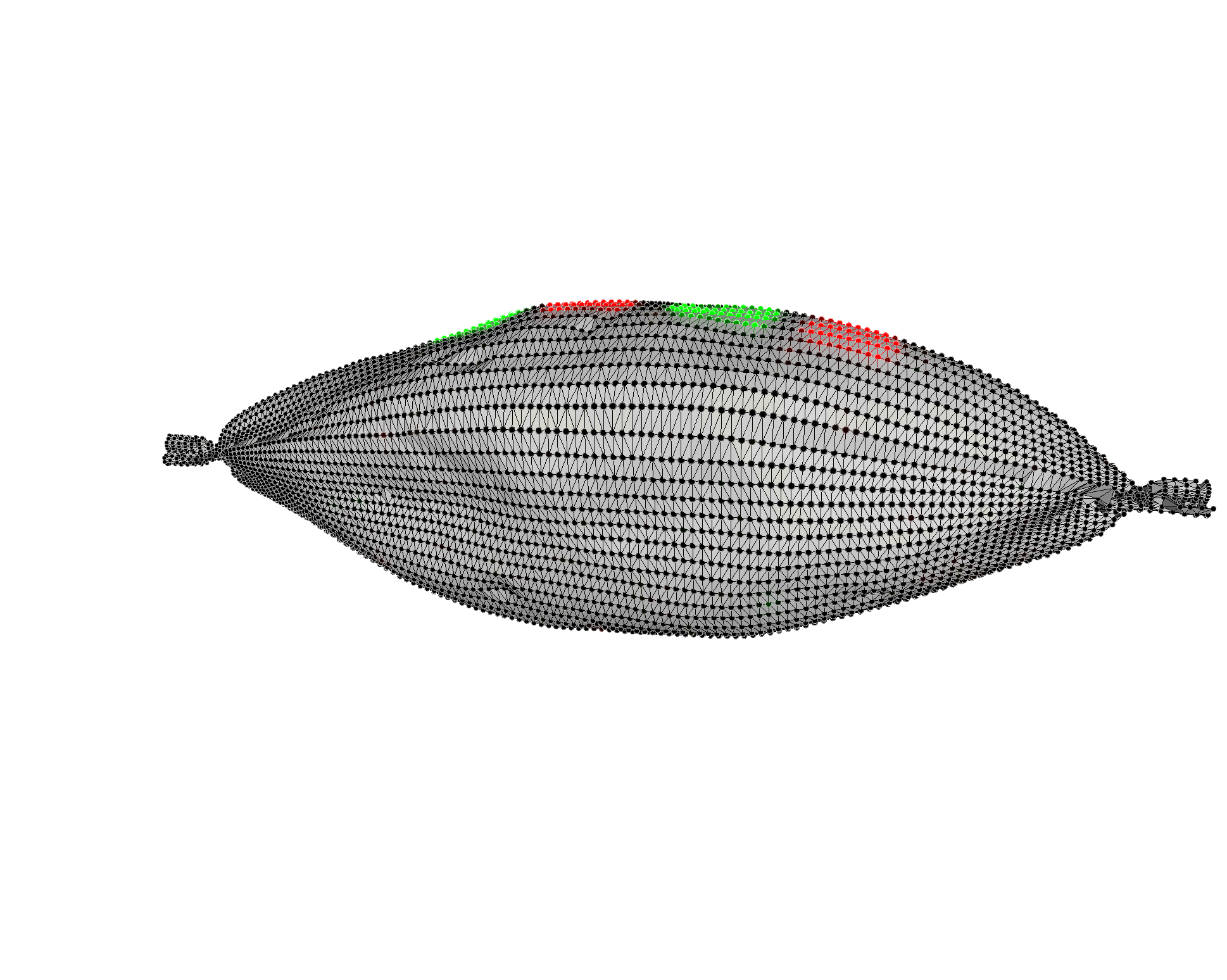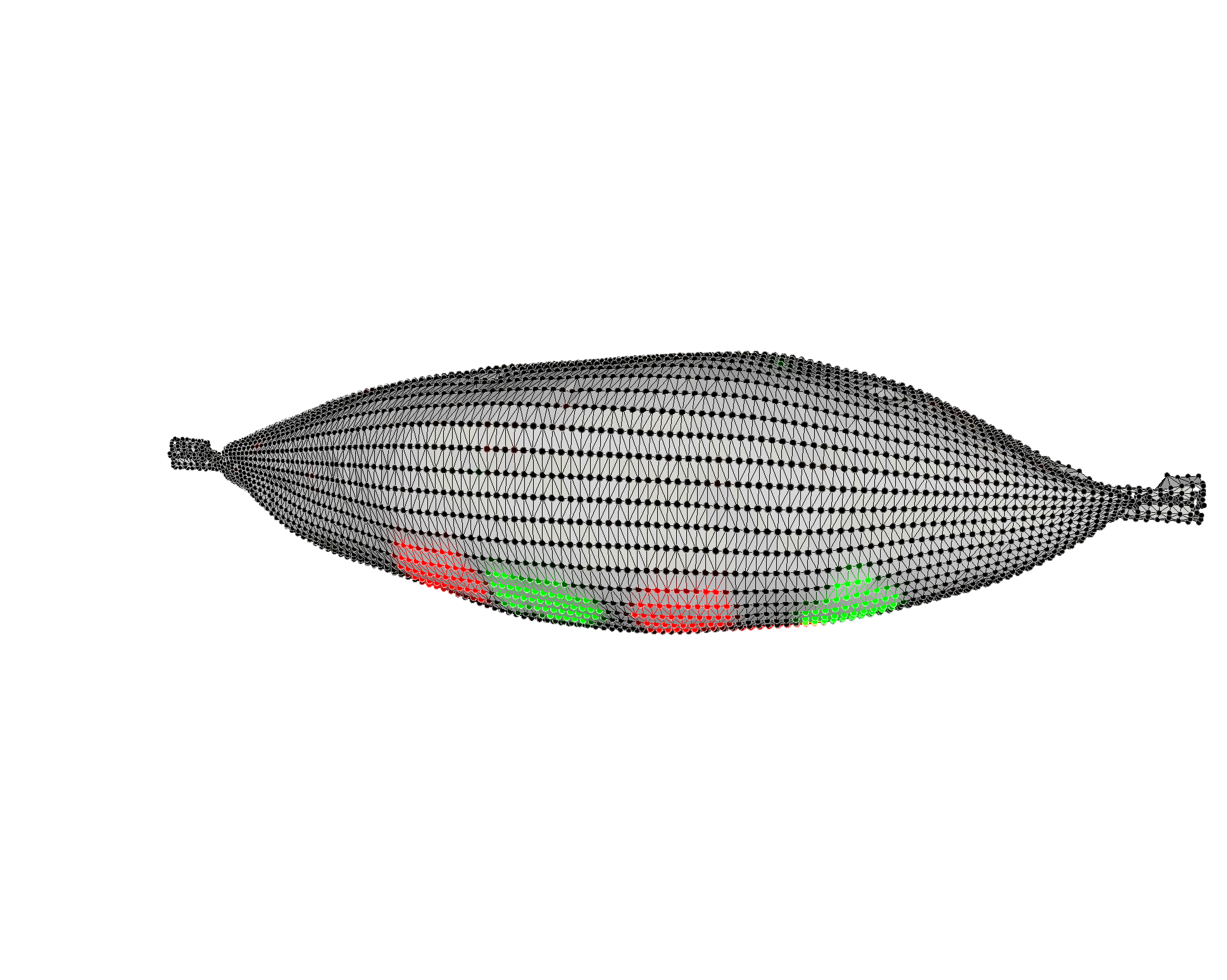

Multiplexed Raman Proteomic Imaging
Failure to fully understand the molecular expression and tumor heterogeneity across a patient’s tumor can lead to administration of ineffective therapies that increase patient morbidity and healthcare costs. The -omics era has made it possible to identify several new molecular markers involved in cancer development, survival, invasion and even predicting treatment response. We currently lack an easy way to obtain high content molecular information while providing high resolution spatial profiling across a patient’s tumor. We are developing an entirely new multiplexed molecular imaging strategy that has the potential to offer both high content molecular expression and spatial profiling in a single histology image. We have created an expansive library of 26 separate SERS nanoparticle batches (Fig. 4A), each bearing a unique spectral fingerprint.

We have also demonstrated their potential to specifically target various biomarkers in the hope of gaining a better understanding of the spatial relationships that exist between a multitude of cell types using Raman imaging. We have also successfully deconvolved a mixture of all 26 SERS NPs in a single imaging pixel both in vitro and in vivo. This opens up new opportunities to efficiently interrogate the heterogeneous molecular expression found within and across patient samples.

Incorporating this innovative approach into the pathology workflow could enable physicians to better understand the patient’s tumor type and stratify patients to receive the most effective therapeutic regimen. This unique histology imaging strategy also has the potential to identify new molecular trends across patients that could be used to predict how aggressive their tumor is or how well the patient is likely to respond to given therapies. These results are an important step toward the translation of this new nano-based multiplexed Raman imaging approach; to provide rapid spatial molecular profiling of a given tumor while enabling a more effective personalized therapy to the patient.
FFPE human tissue sections of ductal carcinoma in situ: left, IHC-stained for HER2, right, Raman image of FFPE tissue section stained with 2-plex mixture of a HER2-conjugated Raman NPs and IgG-conjugated Raman NPs representing specific binding to HER2 and non-specific binding to tissue, respectively. Scale bars=200 μm. The graph depicts DAB optical density plotted as a function of NP binding ratio for the FFPE tissue section.

Center for Translational Medical Engineering
The Zavaleta lab is part of the Center for Translational Medical Engineering at USC. This center focuses on addressing the most urgent medical challenges of our time by introducing new technologies, tools, and approaches that improve and transform clinical solutions. The Center is housed within the new Michelson Building at USC and consists of a team whose expertise spans a range of different biological scales to invent new translational technologies that transform the ways in which we see, predict, and treat medical conditions.

